Abstract
Background
Haploidentical hematopoietic stem-cell transplantation (haplo-HSCT) is associated with an increased risk of graft failure and severe graft-versus-host disease (GVHD). Marrow mesenchymal stromal cells (MSCs) have been shown to support in vivo normal hematopoiesis and to display potent immunosuppressive effects. We launched a multi-center clinical study to examine the safety and feasibility of co-transplantation of MSCs (from third party donors) and haploidentical HSCs into 35 children with severe aplastic anemia (SAA).
Methods
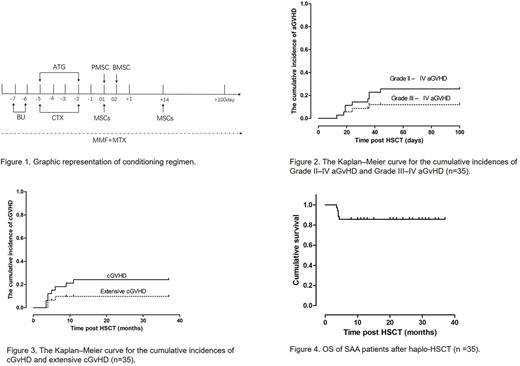
A total of 35 children with SAA were enrolled in this multi-center study between January 2014 and December 2016. All patients met the criteria of HLA-mismatched with ⩾5/10 HLA-matched loci. The conditioning regimen for haploidentical hematopoietic stem cell transplantation consisted of busulfan (Bu), cyclophosphamide and ATG. BM and peripheral blood CD34+ cells were infused intravenously ⩾5×108 cells/kg and ⩾2×106 cells/kg of recipient weight on day 01 and day 02, respectively. MSCs (1×106cells/kg) were administered by venous infusion within 6 h proceeding stem cell infusion on day 01 then on day +14 (figure 1).
Results
Of the 35 patients,16 (45.71%) were considered to have very severe SAA. The patients' median age was 11.5 years (range 3-18). The median mononuclear cell and CD34+ counts were 15.4 × 108/kg and 3.7 × 106/kg, respectively.
All 35 patients (100%) achieved hematopoietic reconstitution and sustained full donor chimerism within 30 days after HSCT. The median time for myeloid engraftment was 14 days (range 10-22 days) and for platelets engraftment 18 days (range 9-36 days).
Almost half of patients (17, 48.57%) experienced aGVHD after transplantation, including 8 (22.86%) with grade I, 5 (14.29%) with grade II, 2 (5.71%) with grade III and 2(5.71%)with grade IV. At 100 days after transplantation, the cumulative incidence of grades II-IV aGvHD was 25.71% and that of grades III-IV was 11.42% (Figure 2). The four patients with grade III or IV aGVHD received additional treatment with methylprednisolone (2 mg/kg/day for 5 days), CD25 monoclonal antibody and the immunosuppressant CsA was changed to FK-506. Four patients were cured, but one patient died.
All 35 patients survived for more than 100 days after transplantation, the majority of them (25, 71.43%) remained disease-free and 8 patients (22.86%) developed cGVHD. Among these cGVHD patients, 5 patients (14.29%) showed limited cGVHD and 3 patients (8.57%) showed extensive cGVHD (Figure 3).
All patients received the conditioning regimen on schedule without encountering any significant transplantation-related, bowel, renal or liver toxicity. No patients experienced infusional toxicity during the infusion of MSCs. Of the 35 patients, 22 developed varying degrees of fever associated with hematopoietic dysfunction which was resolved after treatment with anti-inflammatory medication. In addition, 11 patients developed a CMV infection and 17 developed an EB virus infection and 2 developed an infection with both CMV and EBV. One patient progressed to EBV-associated PTLD and died of viral infection 130 days after transplantation, and another patient progressed to EBV-associated veno-occlusive disease (VOD) and died of viral infection 115 days after transplantation. Two patients developed reversible posterior leukoencephalopathy syndrome (RPLS) died of 120 days and 105 days respectively. Viral infections in the remaining patients were treated with ganciclovir or foscarnet and gamma globulin and, all patients fully recovered.
Patients were followed-up for a median of 22 months (range 3.5-37 months). None of the patients were lost to follow-up. Five patients died within the follow-up period. The causes of death included GVHD (1 patient) and infection (4 patients). The mean survival time was 21.4 months (Figure 4). All 30 of the surviving patients (beyond 8 months) achieved a hematologic complete remission(CR).
Conclusions
Our results indicate that co-transplantation of MSCs with haplo-HSCT is a relatively safe therapy regime for children with SAA. Despite the lack of a comparative control group and limited cases enrolled in the study, our findings support previous studies showing that alternative donor HSCT combined with MSCs infusion could be an effective approach to improve survival rates for patients with SAA.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal